
Medical devices are essential products for patient care. Due to their application in and on the body, there are high requirements from a hygienic-microbiological point of view. These requirements are constantly increasing and are anchored above all in the EU Medical Devices Regulation. We support you with high-quality and individually tailored laboratory services so that you can meet these requirements. Our laboratories are accredited for the testing of medical devices by the DAkkS according to DIN EN ISO/IEC 17025 and GLP certified.
Reprocessing validation
Laboratory tests
The law requires manufacturers of reprocessable medical devices to specify an appropriate reprocessing process. This process must be validated according to ISO 17664-1 and -2. We validate with Dr. Brill + Dr. Steinmann, with our extensive know-how, manual and mechanical reprocessing processes for reusable medical products. We are happy to carry out a procedure suggested by the customer.
Our certications
Biocompatibility
Laboratory tests
Regulations such as the MDR require proof of the biocompatibility of all materials that come into contact with patients or users directly (ISO 10993) or indirectly (ISO 18562). We are happy to accompany our customers on this journey and carry out laboratory tests (e.g. cytotoxicity tests according to ISO 10993-5 on new medical devices or those aged through various reprocessing cycles, ISO 10993-23) in order to create any missing information for the risk assessment. If we are not able to offer the necessary tests in-house, we from Dr. Brill + Dr. Steinmann would also be happy to coordinate projects with third-party laboratories for you. You contact us and we do the rest.

Material compatibility
When designing medical devices, safety aspects must also be taken into account in addition to functional issues. For example, the expected lifespan should not only apply to use on patients, but also with regard to the physical and chemical processes to which the product is likely to be exposed during reprocessing. For this purpose, we can check the material compatibility of a chemical process for you on a whole range of different materials. In addition, Dr. Brill + Dr. Steinmann can also discuss very practical questions with you. If, for example, you would like to know how the material of your specific product changes after several cycles of chemical treatment, we would be happy to simulate this for you and then test how practical treatment might affect cytotoxicity (ISO 10993-5).
Hygiene in production and laboratory
Hygiene monitoring
We are also available for hygienic monitoring at Dr. Brill + Dr. Steinmann. Our experienced field staff will be happy to come to you and take samples to determine the germ count of surfaces (swabs and agar plates) and the air (active/air samplers and passive/sedimentation plates). Included in the service package is the incubation and evaluation of environmental samples. Germ identification is also possible. If there is interest, this data can then be used by our partner laboratories to systematically track contamination. This way you have everything in one database and can combat possible sources of contamination more effectively. You can of course also use this service in conjunction with disinfection validation. Please feel free to contact us!
Hygiene in the health sector
In cooperation with the company IDEXX, we offer the introduction and monitoring of hygiene management systems for veterinary clinics and veterinary practices. The services include inspecting the facility, preparing status reports with suggestions for improvement, training employees, and developing an individual hygiene manual. If the hygiene quality meets our requirements, we will issue a hygiene certificate upon request, which you can use on your website, in your rooms, etc. for marketing purposes.
Hygiene in production and laboratory
Disinfection validation
When there is a change in the cleaning and disinfection procedure in the production process, pharmaceutical companies have to carry out complex validations to prove that the new or changed process works. With the identification of in-house germs that could be isolated in production, in combination with surface materials from practice, the disinfection performance of a newly introduced disinfectant can be validated in the laboratory. We validate your cleaning and disinfection processes in your production, e.g. if there are changes to the process or a new production area is put into operation. We are happy to contribute our expertise in the areas of disinfectant efficacy testing and the reprocessing validation of medical devices. We can at Dr. Brill + Dr. Steinmann can validate surface disinfection process based on EN 13697 without mechanics but also with mechanics and the use of e.g. B. Validate wipes based on EN 16615 for you. We are also happy to collect the in-household germs relevant to you on site as part of a hygiene monitoring service package. So please feel free to contact us with your inquiry.
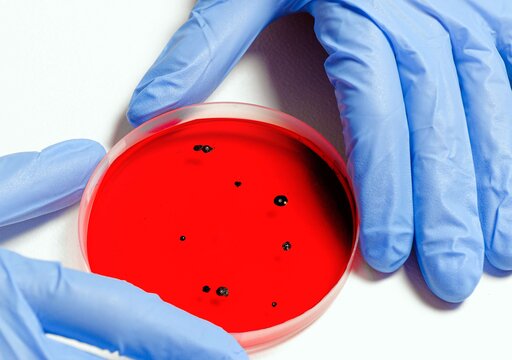
Hygiene in production and laboratory
Hygiene service bioindicators
Are you looking for suitable bioindicators for your cleaning and disinfection device (for endoscopes), washing machines or dishwashers? We at Dr. Brill + Dr. Steinmann design bioindicators for a wide range of applications and of course also offer you microbiological evaluation after their use (DIN EN ISO 11138, DIN EN 13060, DIN EN ISO 18472, DIN EN 13060). So please feel free to contact us and ask about the different service packages we have established for you.
Medical products containing biocides
Disinfectants and wound dressings
Is your medical device an instrument disinfectant? Please feel free to contact us because we test the efficacy of your disinfectants, for example according to EN 14561/2/3 and EN 17111. You can also have your cleaning products tested by us.
Is your product intended for antimicrobial wound treatment? We can help here too and test according to EN 17854, for example. In addition to the classic standard testing, we have also established other unique procedures on the topic of wounds (e.g. 3D skin model), which you as a customer of Dr. Brill + Dr. Steinmann can benefit from.
Medical products containing biocides
Biofilms
The fight against biofilms is a major challenge for many disinfectant manufacturers given regulatory uncertainties on the one hand and technical challenges on the other. We at Dr. Brill + Dr. Steinmann however, through the development and establishment of various models with regard to biofilms, have a great deal of expertise that you can draw on. Benefit from various technical options that can provide useful insights in the context of biofilms. Among other things, we can offer you the following specific services for your medical device:
- ISO/TS standard 15883-5: 2006, Annex F or the method according to Pineau et al. 1997 (e.g. for flexible endoscopes)
- House method according to Brill et al. 2018 (e.g. for catheter rinsing solutions or equipped catheters)
- Method according to Sturmer et al. 2021, 2022 (e.g. for biofilms in wounds)
However, processes often have to be developed or adapted specifically for your application and your product. We would be happy to do that with you. So if you have a need, we will be happy to inform you in detail.
Clinical studies on test subjects with microbiological endpoints
The offer in our phase 1 study center in Hamburg refers in particular to studies to prove an antimicrobial effect of products that are applied to the uninjured skin or mucous membrane as well as to studies on the skin microbiome and its change through the application of products. Endpoints can be the total microbial count or the change in previously artificially applied organisms through to microbiome research. Are your products “microbiome friendly”? If not, perhaps it's time.
We have been carrying out tests for over 20 years, particularly on the efficacy of hand disinfectants and skin antiseptics in accordance with standard norms. With Ralf Freese, we have now brought an investigator and Dr. Saskia Borregaard, a GCP expert for the regulatory framework, into the team. Both have over 15 years of experience in planning, conducting and evaluating clinical trials.
This enables us to offer everything from a single source for medical device and drug studies at GCP (Good Clinical Practice) level with an up-to-date SOP system, from the idea, study design with meaningful, helpful and realistic microbiological endpoints by our expert Dr. Florian H. H. Brill, writing the study protocol, submission to the ethics committee, recruitment and education of subjects, conducting the study, sampling of subjects, microbiological evaluation and reporting.
If you are interested in participating in the studies as a volunteer, please contact us HERE.
We are also open to partial steps in which our clinical-microbiological experience is helpful for you. We are available for consultation in the context of study design or as a microbiology laboratory to take over the evaluation of samples in the context of clinical trials at other study centers and, for example, to provide sampling kits. We are of course also available as a study center including a microbiology laboratory if you can and want to map the regulatory part differently.
On request, we can take over the complete organization from the idea, through the design and submission to the ethics committee, the execution of the study to the evaluation and interpretation of the data. Of course, we are also happy to offer microbiological services in the context of clinical studies at other study centers.
We look forward to receiving your inquiries, which will be dealt with personally by Dr. Florian H. H. Brill and his team.

Training
We offer a lot of interesting training courses on the topics of disinfection, cleaning, sterilization, general microbiology and hygiene, reprocessing of medical devices and hospital hygiene. Of course, we also put together courses specifically tailored to your requirements. The duration of a course can be from 30 minutes to 4 days, depending on your wishes and needs.

Portfolio
Excerpt
|
EN DIN EN 1040 DIN EN 1275 DIN EN 14347 DIN EN 13727 DIN EN 13624 DIN EN 14348 DIN EN 17126 DIN EN 14476 |
EN DIN EN 16615 DIN EN 17111 DIN EN 16777 DIN EN 14561 DIN EN 14562 DIN EN 14563 DIN EN 16616 DIN EN 17854 |
Others Ph. Eur. 2.6.12 Ph. Eur. 2.6.13 Ph. Eur. 5.1.3 Ph. Eur. 5.1.2 |
ASTM ASTM E1053 ASTM E2314 ASTM E1837 ASTM D7907 |
ISO ISO 10993-5 ISO 15883-5 ISO 11737-1 ISO 22196 |
