
News Archive
You will find the latest information about our company and industries here. Please feel free to contact us with any questions and suggestions relating to our topics or content!

Topic “Multi-resistant pathogens” at the Online Update Hygiene und Infektionsprävention #17
At the free Online Update Hygiene und Infektionsprävention #17 on the topic of “Multiresistente Erreger" (Multi-resistant pathogens) on 8 April 2025, Dr. Florian H. H. Brill will give a lecture in German entitled “Wirksamkeit von Flächendesinfektionsmitteln gegen klinische Isolate Multiresistenter Erreger" (Effectiveness of surface disinfectants against clinical isolates of multi-resistant pathogens). Use the Online Update #17 to find out about important questions on the topic of “Multi-resistant pathogens” and to exchange ideas with experts from clinics, research, politics and industry. You can find all information, including on the two other presentations, as well as for your registration under the following link:
#17 - Multi-resistant pathogens
The Online Update Hygiene und Infektionsprävention is organized as a cooperation event within the framework of HIHeal by Life Science Nord, the Department of Hospital Hygiene of the University Medical Center Hamburg-Eppendorf and DR. BRILL INSTITUTES.

BIOFILMS 11 conference with lecture by Prof. Dirk Bockmühl
“Effects of Antimicrobial Compounds in a Complex Biofilm Sink Drain Model” is the title of the presentation Prof. Bockmühl will give at BIOFILMS 11. The conference will take place from 13 - 15 May 2025 in Cardiff (UK). Prof. Bockmühl's presentation is part of the session “Preventing Biofilms: Design through knowledge-based approaches” on Day 2, Wednesday, 14 May 2025, 09:00-09:40.
The conference is showcasing the broad scientific and societal opportunities and challenges of microbial biofilms. A key objective is to capitalise on the interdisciplinary nature of this research topic by fostering discussion and catalysing the exchange of ideas across boundaries.
The entire program and all further details, also for your registration, can be found here: BIOFILMS 11
In addition to Prof. Bockmühl, Dr. Britta Brands from the Dr. Brill + Prof. Bockmühl GmbH Institute for Applied Hygiene will also be in Cardiff. You are therefore welcome to use the conference for a personal exchange with our colleagues on site. To arrange an appointment, please contact us by e-mail HERE.
We look forward to seeing you again or getting to know you for the first time in Cardiff!
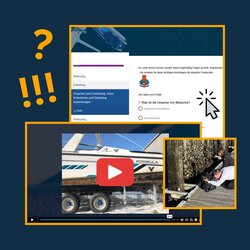
E-learning course: Biofouling management for recreational craft
The e-learning course “Biofouling Management for Recreational Craft” is now available online. The course was created on behalf of the Federal Maritime and Hydrographic Agency (BSH, Bundesamtes für Seeschifffahrt und Hydrografie) by our Institute for Antifouling and Biocorrosion on Norderney, in collaboration with Alex Jamil as film producer and Claudia James from HYGIENE COMPASS for making the course available on their online platform. For boat owners, clubs, associations and authorities, it serves as an awareness-raising, preventive measure with regard to the spread of non-native species, as well as to minimize the entry of harmful substances from antifouling coatings and emissions. In five chapters, the causes, development and effects of biofouling, protection against biofouling, alternative anti-fouling systems, cleaning technologies, biofouling management plans, regulations and further information options such as Fouling Atlas and Blue Angel are shown. Each chapter consists of a film followed by a questionnaire. The films last between 5 and 9 minutes, the questionnaires consist of 4 to 12 questions in multiple choice mode. The entire course takes about 45 minutes, but can also be completed in several stages. Participation is free of charge for everyone!
A short-term goal is to train “biofouling experts” in as many marinas as possible, who will inform users or club members about existing regulations and provide support, for example, in the choice of antifouling system (AFS) or the cleaning and maintenance of the AFS.
The long-term goal is to reduce the discharge of pollutants into the waters (inland, North Sea and Baltic Sea), CO2 emissions and to minimize the introduction and spread of non-native species without negatively affecting the protection of fouling.
Detailed BSH press release in German languange - here: Link
Click here to go directly to the course in German language: E-Learning-Kurs "Biofouling-Management für Sportboote"
A version of the course in English language will follow within the next weeks.
We thank the partners involved:
BMDV-Expertennetzwerk
DSV Deutscher Segler-Verband
DMYV Deutscher Motoryachtverband
HYGIENE COMPASS
SportJobs Bildung und Bewegung

DGKH: Symposium on 16-17 May 2025 in Essen - Four lectures with participation of our institute
At this year's symposium of the German Society for General and Hospital Hygiene (Deutsche Gesellschaft für Allgemeine und Krankenhaus-Hygiene - DGKH) in Essen, our institute will be involved in the following four presentations (in chronological order):
17 May 2025, 08:00-09:30, Room Ruhr
Reinigung, Desinfektion, Sterilisation - Aufbereitung von Medizinprodukten
(Cleaning, disinfection, sterilization - reprocessing of medical devices)
F. Brill (Hamburg):
Automatisierung der Aufbereitung semikritischer Medizinprodukte und Nachweis der Effizienz der Aufbereitungsverfahren am Beispiel transvaginaler Ultraschallsonden inkl. praktischer Implikationen
(Automation of the reprocessing of semi-critical medical devices and proof of the efficiency of the reprocessing procedures using the example of transvaginal ultrasound probes, including practical implications)
M. Wehrl (Krefeld):
Feldstudie 2: Bestimmung der Reinigungswirkung in Endoskopkanälen mittels Flush-Brush-Flush (FBF)-Verfahren
(Field study 2: Determination of the cleaning effect in endoscope channels using the flush-brush-flush (FBF) method)
17 May 2025, 12:00-13:30, Room Ruhr
Reinigung, Desinfektion, Sterilisation - Reinigung und Desinfektion
(Cleaning, disinfection, sterilization - Cleaning and disinfection)
K. Konrat (Berlin):
Einfluss von Zellkulturmedien und -zusätzen auf die viruzide Wirksamkeit chemischer Desinfektionsmittel
(Influence of cell culture media and additives on the virucidal efficacy of chemical disinfectants)
D. Paulmann (Bremen):
In-vivo Wirksamkeit von Händedesinfektionsmitteln gegen das Adenovirus in Anlehnung an EN 17430
(In vivo efficacy of hand disinfectants against the adenovirus based on EN 17430)
Regarding Field Study 2, we are a member of the methodology group that conducted this study. Dr. Andreas Kampe represents us there. And the presentation “Influence of cell culture media and additives on the virucidal efficacy of chemical disinfectants” is a joint project between our institute and the RKI.
We would of course be delighted to welcome you in Essen - and you are also welcome to use the symposium for a personal exchange with our colleagues on site. To arrange an appointment, please contact us e.g. by email HERE.
The entire conference program and all further information for your registration can be found at: https://kongress.krankenhaushygiene.de/
Conference venue:
Messe Essen, Congress Center Ost
Messeplatz 1, 45131 Essen, Germany

Dr. Florian Brill represents the laboratory sector on the new DIN SME Council
Dr. Florian Brill has been appointed to DIN's new SME Council, where he represents the testing and laboratory sector as Vice President of the German Association of Independent Testing Laboratories (VUP). The SME Council is a cross-thematic and cross-industry advisory body that supports the operational management level of DIN with regard to the interests of small and medium-sized enterprises (SMEs) in standardization. The aim is to facilitate the participation of SMEs in standardization processes and to ensure that their interests can be represented in an effective and balanced manner, regardless of their sector and size. The SME Council's advisory focus is on
• Identification of SME interests in standardization
• Simplifying the standardization process, especially for cross-cutting issues
• Simplification of standards and the reduction of their partial complexity for application in SMEs
• Development and review of supporting instruments
• Raising external awareness of important standardization topics
• Recruitment and qualification of standardization experts
• Development of SME training modules
The 25-member advisory board will hold its inaugural meeting on 19 February 2025.
DIN - Medium-sized companies: HERE

Newly established IBRG methods
Do you have a PT6 product and need an efficacy test in accordance with the ECHA guideline? We are pleased to inform you that we now have this service in our program for you too. IBRG PDG16-001 and IBRG PDG16-007 can be ordered now. IBRG P16-001 will also be available soon, so that you can cover all PT6 applications in tier 1 with tests from us. We look forward to your orders and requests, e.g. by email: HERE.

News from the expert groups - Our engagement continues!
In recent weeks, we have again been present at various expert groups on standardization.
Dr. Tilman Gehrke has been supporting the development of a test method for testing mortar and concrete formulations for their resistance to biogenic sulphuric acid corrosion, which occurs particularly in wastewater pipes and destroys the corresponding pipes, for over eight years now. The method is based on work carried out in the 1980s at the University of Hamburg by Professor Dr. Wolfgang Sand, Dr. Gehrke's doctoral supervisor, and also by Dr. Florian H. H. Brill. The next meetings of the DIN and CEN working groups took place here. We have been offering this procedure for many years with the Fraunhofer Institute UMSICHT and Dr. Holger Wack, with whom we maintain a very successful cooperation (see here: LINK).
Dr. Florian H. H. Brill chaired the meeting of Strategic Working Group 5 of CEN TC 216 "Disinfectants and Antiseptics". The restructuring of the group is working. We have established various task forces that have started their work, e.g. Airborne disinfection, Biofilms, Ring trials, Resistance. A new task force on harmonization is in the start-up phase and is intended to significantly reduce the complexity in CEN TC 216 and at the same time promote harmonization. To this end, a task force has been set up to optimize reproducibility in the test principle of surface disinfection without mechanics. There was also a meeting of Working Group 1 "human medicine" in CEN TC 216 and the corresponding German mirror committee at DIN: Dr. Florian H. H. Brill also represented us here, and we are heavily involved in the following projects:
- Anna Ulatowski and Dr. Florian H. H. Brill: Revision of EN 14348 based on our paper https://pubmed.ncbi.nlm.nih.gov/33582203/
- Dr. Dajana Paulmann and Dr. Britta Becker: Development of the standard for the 4-field test in virucidal testing. See our paper https://pubmed.ncbi.nlm.nih.gov/31346462/
- Dr. Dajana Paulmann and Birte Bischoff: Revision of EN 14476 with the increase of the test temperature to 25 °C for hand hygiene products
- Dr. Florian H. H. Brill and Dr. Britta Becker: Replacement for the poliovirus under pressure from the WHO. We have already published a paper on this topic: https://pubmed.ncbi.nlm.nih.gov/35033614/
- Henrik Gabriel and Anna Ulatowski: Revision of EN 14561/2/3
- Dr. Jan Klock and Anna Ulatowski: Revision of EN 16615, also based on our paper https://pubmed.ncbi.nlm.nih.gov/37598903/
WG 3 "Food, consumers, industry, institutional sector" and the mirror committee at DIN also had their meetings, in which Dr. Florian H. H. Brill took part. We are involved in the development of the following standards:
- Dr. Britta Becker and Dr. Dajana Paulmann: Development of EN 17914, EN 17915
- Dr. Florian H. H. Brill and Anna Ulatowski: Method for efficacy testing of surface disinfectants with mechanics.
The ISO TC 330 "Antimicrobial Surfaces" also had both the Working Group 2 meeting, where we are involved in the development of a test method for the efficacy evaluation of antiviral hand contact surfaces with a novel "touch transfer method". In 2021, we published an exciting paper on banknotes, coins and credit cards: https://pubmed.ncbi.nlm.nih.gov/34337354/. The first draft standard was discussed here. The plenary meeting also took place in December to discuss the strategic direction. Dr. Florian H. H. Brill represented us at both meetings.
We are also active at ECHA level. Heike Schimmelpfennig represented us for the first time at the various committee meetings of the Biocidal Product Committee (BPC).

Update completed: Study "Sustainable solutions against biofouling - a stakeholder overview"
Our Institute for Antifouling and Biocorrosion on Norderney has updated the market transparency study “Biofouling - Solutions against biofouling on ship hulls” (Biofouling - Lösungsansätze gegen Biofouling an Schiffsrümpfen). The Competence Center GreenShipping Niedersachsen, together with the Maritime Cluster Norddeutschland e.V. (MCN) and EurA AG, had compiled this German-language market overview for the first time in 2020. Now, Bernd Daehne (Director of Antifouling Research Station) and his team have updated this overview. It presents players who offer solutions to combat biofouling on ship hulls or who are conducting research in this area. Companies, universities and institutions can thus find suitable cooperation partners for various projects in the field of anti-fouling technologies and techniques more quickly and easily. The aim of the overview is to increase market transparency and to support research and development, thereby driving it forward in a faster and more targeted manner.
The market transparency overview "Nachhaltige Lösungsansätze gegen Biofouling - eine Stakeholderübersicht" (Sustainable solutions against biofouling - a stakeholder overview) is made available for download on the websites of the Maritimes Cluster Norddeutschland e.V. and the Competence Center GreenShipping Niedersachsen. LINK

Arab Health 2025: Please visit us!
Arab Health will take place in the Dubai World Trade Centre from 27 - 30 January 2025. Dr. Kerstin Walendy-Gnirß will again be present. Please contact us for an appointment there and use the opportunity for a personal exchange. We would be very pleased to meet you again or to get to know you in Dubai!
At the same time, we would like to thank all of you who visited us at MEDICA 2024 in Düsseldorf in November and at Biocides Europe 2024 in Vienna in December. As always, it was a great pleasure for us to maintain, refresh and make so many new contacts there.
Link to the appointment request by e-mail: HERE.

Big anniversary a great success: These were some of the highlights of the 10th CAHMV 2024 - Save the date 2025!
On 28 and 29 November 2024, Dr. Florian H. H. Brill and Alexander Brux welcomed over 100 international guests to the 10th anniversary of the Conference of Applied Hygiene, Microbiology and Virology in Hamburg. The conference was organized for the first time in cooperation with our partner BioGenius, who celebrated their 20th company anniversary at the same time.
The following highlights stood out in a rich scientific program:
- The panel discussion “25 years of biodical regulation”, which was held for the first time
- The interesting perspective of hospital management on infection prevention from our key note speaker Professor Dr. Ojan Assadian
- The session “Hot topics infection prevention & control” with the talk of Dr. Toni Meister on (re-)emerging viruses
- The wonderful keynote lecture from Professor Dr. Rasmus Leistner on the successful integration of probiotic-based cleaners in a hospital environment
- The traditional Keynote lecture on day 2 from Dr. Florian H. H. Brill on developments in the field of efficacy testing of antimicrobial products
- The two sessions on the hot topics of resistance development through biocides and efficacy testing in biofilms were held in parallel and did not make it easy for participants to decide which session to attend. The presentation on the development and testing of resistance by Professor Dr. Laurent Piorel was just as much a highlight as the presentation of the new COST action “Regulatory Tool Box” (LINK) on biofilm methods by Dr. Paulina Rakowska.
We received very positive feedback from the participants. They liked the scientific content, but also the opportunity to network, e.g. during the harbor tour on Thursday evening. We are therefore already looking forward to the next, then 11th Conference of Applied Hygiene, Microbiology and Virology, which will take place on 27 and 28 November 2025, again in Hamburg (see "Events": HERE). Please save the date in your calendar now.

Online events in spring 2025: “Environmental Risk Assessment of Biocides” and “Antifouling”
At the online conference “Environmental Risk Assessment of Biocides” in late March, DR. BRILL INSTITUTES Heike Schimmelpfennig, Dr. Brill Regulatory Services GmbH, and Bernd Daehne, Dr. Brill + Partner GmbH Institut für Antifouling und Biokorrosion, will be among the speakers. Heike Schimmelpfennig will speak on the topic “Comparison of Environmental Risk Assessments across different legislative areas”, Bernd Daehne's presentation title is “Non-biocidal alternatives to antifoulings and how they compare to the biocides in terms of performance”. Registration is already open and we look forward to your participation. All further information can be found here: LINK
Organizer: Die Akademie Fresenius GmbH
Furthermore, on February 6, you will have the opportunity to take part in the webinar “Antifouling” of the magazine FARBE UND LACK, also with Bernd Daehne as speaker. Topic (in German language): “Antifouling - basic knowledge in 120 minutes”. Registration is already open. All information can be found here: LINK

New: Tests with sea pipes according to ASTM D6990-20 (evaluation)
Dr Brill + Partner now also offers fouling tests in pipes. More and more applications require pipe systems through which natural seawater flows (cooling systems, LNG, hydrogen production, seawater heat pumps). However, biofouling can constrict the pipe cross-section, which reduces the flow and thus impairs the effect, leads to increased energy consumption and, in the worst case, even to system failure. Dr Brill und Partner has developed equipment for simulated field tests in Norderney harbour to test anti-fouling methods designed to prevent this. Here, simulated practical tests can be carried out under real-life conditions in pipelines with a defined seawater flow rate during the summer season from April to September. Analyses are carried out using endoscope cameras and test plates in accordance with ASTM D6990-20. During the test phase, customers are regularly informed about fouling development through inspections and the subsequent provision of photos and videos by email or cloud. Link: Dr. Brill + Partner GmbH Institute for Antifouling and Biocorrosion