
News Archive
You will find the latest information about our company and industries here. Please feel free to contact us with any questions and suggestions relating to our topics or content!

6th Hull Performance & Insight Conference (HullPIC)
From 30th Aug. 2021 to 01st Sept. 2021, the Hull Performance & Insight Conference (HullPIC) will be held in Pontignano, Italy.
The conference will focus on presentations and discussion sessions on the following topics:
- ISO 19030 and beyond
- sensor technology
- human factors in reporting & understanding performance
- information fusion
- big data
- uncertainty analysis
- hydrodynamic models
- business models
- cleaning
- technology
- coating technology
- ESDs
Bernd Daehne from our Institute for Antifouling and Biocorrosion will give a presentation entitled: "ROBUST: Integrated Coating & Cleaning Concept for Offshore Structures" at the conference on 01.09.2021 at 13:55. More information about the conference can be found HERE.

Dr. Brill + Partner listed as GLP testing facility
Our institute has already been certified as a GLP testing facility since June 2020. This certification is valid for the test sites Hamburg and Bremen (To the certifications). Now we have also been included in the German directory of test facilities / test sites (July 2021) (Directory GLP test facilities) The certification applies to the categories 2 tests for the determination of toxicological properties, 3 tests for the determination of mutagenic properties (in vitro and in vivo) and 9 for microbiological and biochemical testing of cleaning, disinfection and sterilization processes. We would like to thank all our employees and partners and are looking forward to your request.

6th International Conference on Prevention & Infection Control (ICPIC)
This year's "International Conference on Prevention & Infection Control (ICPIC)" will take place in Geneva from 14.09.2021 to 17.09.2021.
Dr. Florian H. H. Brill is also involved in this year's conference with two presentations.
15.09.2021, 14:35-14:40 - Virucidal efficacy of an ozone-generating system for automated room disinfection (Dr. Florian H. H. Brill)
16.09.2021, 10:00-10:12 - Development of practice-simulation in vivo efficacy test method for alcohol-based hand rubs based on EN 1500 and ASTM E2755 (Dr. Florian H. H. Brill)
You can find more information about ICPIC HERE

Hamburg Copenhagen Business Forum
The Hamburg Chamber of Commerce cordially invites you to the 4th "Hamburg Copenhagen Business Forum". The Hamburg Chamber of Commerce is organizing the forum together with the German-Danish Chamber of Commerce and Dansk Industri this year under the motto "Germany and Denmark - Joining Forces for Green Growth".
In addition to discussion forums on renewable energy, the green real estate industry, urban mobility and the digital health industry, the conference offers business representatives from both countries the opportunity to network and expand business relations in their respective neighboring countries. Among others, Hamburg's Senator for Economy and Innovation, Michael Westhagemann, and the Danish Minister of Transport, Benny Engelbrecht, will participate in the forum. In addition to the full-day conference on August 25, 2021, the following day will include a tour program to the House of Green, the Middelgrunden Offshore Wind Farm, and the Amager Resource Center including Copenhill.

New Publication: A realistic transfer method reveals low risk of SARS-CoV-2 transmission via contaminated euro coins and banknotes
On our "Publications" page you will find a new publication on the transferability of SARS-CoV-2 to banknotes and coins

Online-Update Hygiene and Infektionsprävention: COVID-19 #6
More than any other crisis, the pandemic affects all generations, all social groups and social, economic, political and cultural life worldwide. It is being felt everywhere, albeit to varying degrees.
In this free online update, we would like to offer you the opportunity to find out about important questions on the topic and to exchange ideas with our experts from the clinical, research, political and industrial sectors.
Speakers:
Korinna Hennig
NDR science journalist and host of the podcast "Coronavirus Update
Science communication in times of crisis
more to follow...
The online update is a cooperation event with Dr. Brill + Steinmann, MedWiss4you and HIHeal.
Life Science Nord GmbH coordinates the HIHeal network as the first so-called "Hamburg Cluster Bridge Project", whose funding is provided by the European Regional Development Fund (ERDF) and the Free and Hanseatic City of Hamburg. Another topic area within the project is eHealth, coordinated by Gesundheitswirtschaft Hamburg.
Click HERE to register

Dr. Florian Brill is VUP President
On June 17th, 2021, Dr. Florian Brill was elected as the new president of the VUP for the next two years. He was first elected to the VUP Presidium in 2016. Dr. Florian Brill is also currently heading the project group "VUP 2025" and represents the VUP in numerous external bodies.
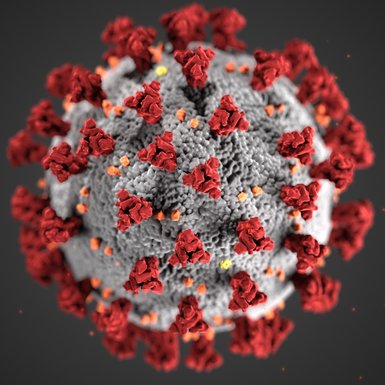
Human Coronavirus established!
We are happy being able to extend our testing service for you. As of now we can offer activity tests of disinfectants, antiseptics, and antiviral surfaces with the human Coronavirus, strain E229 in our testing laboratory in Bremen. The team established the virus in the lab during the last months and now it is validated.
In case you are interested, please contact us to get an individual quotation.

Vaccinations at the company doctor
Finally, the time has come. As of the 7th of June, Corona vaccinations will be available through the company doctor. Our colleagues and their spouses have already been able to register for the vaccination. The vaccinations will take place in the practice of the company doctor, there will not be a collective vaccination center with the surrounding companies.

Kompendium Flächenhygiene
On May 19th, 2021 the book "Compendium Surface Hygiene" was published by Günter Kampffolgen. Dr. Florian H.H. Brill and Dr. Maren Wodrich from the institute are part of the team of eleven authors.
"Dieses Buch ist als Standardwerk für die wesentlichen Aspekte der Flächenhygiene in allen Bereichen der Versorgung von Patienten und Heimbewohnern konzipiert. Erkenntnisse aus der internationalen wissenschaftlichen Fachliteratur werden umfassend ausgewertet und dargestellt, Fallbeispiele, Infoboxen und Abbildungen erleichtern deren Lesbarkeit. Zahlreiche Tabellen ermöglichen eine intensivere Beschäftigung mit Einzelergebnissen. Mit einem Fazit am Ende eines jeden Kapitels werden die für die Praxis wesentlichen Erkenntnisse komprimiert zusammengefasst. Ein Team aus insgesamt 11 Autoren befasst sich im ersten Teil des Buches mit der Kontamination von Flächen, den Übertragungswegen, Flächen-assoziierten nosokomialen Infektionen, dem OP-Bereich, der unmittelbaren und erweiterten Patientenumgebung, Stethoskopen, mobilen elektronischen Geräten, der praktischen Durchführung von Reinigung und Desinfektion, dem Schutz der Mitarbeiter bei der Durchführung der Flächendesinfektion, den verschiedenen Tuchspendersystemen sowie der Qualitätssicherung in der Flächenhygiene. Im zweiten Buchteil werden die Prüfmethoden zur Bestimmung der Wirksamkeit, die antimikrobielle Wirkung der häufigsten chemischen Wirkstoffe - einschließlich Kupfer und Photodynamik - sowie Möglichkeiten zur Reduktion des bakteriellen Selektionsdrucks in der Flächendesinfektion beschrieben („Antimicrobial Stewardship“)." (Abgerufen am 22.05.2021 von https://tredition.de/autoren/guenter-kampf-36426/kompendium-flaechenhygiene-hardcover-154960/)

Dr. Brill + Partner GmbH is partner laboratory of the School of Life Science at UKE
Every year on August the 1st the training for Biological-Technical Assistant starts at the School of Life Science of the UKE. The two-year training is aimed at anyone with a keen interest in biology and, in particular, in a wide range of laboratory activities.
The first 9 months of the training focus on theory. This is followed by a year of gaining practical experience. In 3 internships of 4 months each, the students get the chance to get to know different institutes, such as us, the Dr. Brill + Partner GmbH Institute for Microbiology and Hygiene.
very year on August the 1st the training for Biological-Technical Assistant starts at the School of Life Science of the UKE. The two-year training is aimed at anyone with a keen interest in biology and, in particular, in a wide range of laboratory activities.
The first 9 months of the training focus on theory. This is followed by a year of gaining practical experience. In 3 internships of 4 months each, the students get the chance to get to know different institutes, such as us, the Dr. Brill + Partner GmbH Institute for Microbiology and Hygiene.
Lukas Kuhn completed his training at the School of Life Science of the UKE from 2017 - 2019. At that time, he did his first internship at our institute on Norderney. After his training, Lukas found his way back to us and has been working with us on Norderney ever since. There he is responsible for technology. Lukas keeps existing test stands running, carries out minor repairs and is involved in the development of new test methods and equipment.
"The work here on Norderney is special in that every day is different here. You work a lot outside at the harbor and on the beach and are dependent on the weather and tides there. My tasks here are very varied, from evaluating experiments to image processing and manual work. In addition, there is a very pleasant working atmosphere with top colleagues."
Applications to the School of Life Science for the training start August 2021 are still possible until May 31.
We look forward to sharing our knowledge and experience with all future interns.

11. eHealth-Lounge - "The Future of Hygiene"
Epidemics of influenza or the current Corona pandemic are impressive examples of how microorganisms can spread within a very short time and what consequences such spreads can have for a society. Viable hygiene concepts and preventive measures for the prevention of infections are therefore relevant in all areas of life. New, traceable and safe infection prevention solutions must be developed and implemented in hospitals as well as in inpatient and outpatient facilities and services.
Which (digital) solution options for better hygiene protection already exist and how can these solutions be transferred to other areas and sectors? What can we learn from the requirements of the pandemic? How will we deal with the issue of hygiene in the future? On the occasion of the International Day of Hand Hygiene, the eHealth Network Hamburg of Gesundheitswirtschaft Hamburg GmbH takes up these and other questions in our next online event and invites you to the 11th eHealth Lounge - "The Future of Hygiene".
Date: May 5, 2021
Time: 4:00 p.m. to 5:30 p.m.
You can find more information about the event HERE